Going Organic
 Organic Chemistry should be chemistry made only with natural ingredients, without those nasty chemicals, right? Well, that's not too far off. It started out as the study of stuff coming from living organisms. That stuff seemed distinctly different from the rest of chemical compounds – it was sort of softer and smoother, more "organic".
Organic Chemistry should be chemistry made only with natural ingredients, without those nasty chemicals, right? Well, that's not too far off. It started out as the study of stuff coming from living organisms. That stuff seemed distinctly different from the rest of chemical compounds – it was sort of softer and smoother, more "organic".
Until the early 1800s, those "organic" compounds only came from nature. So the great minds of the time, being as arrogant then as they are today, decided that will always be that way.
Some even went farther and made up a reason for it – a woo “vital force” that only living things possess.
That intellectual triumph was tragically undercut in 1828 by one Friedrich Wöler, who quite carelessly
prepared urea in the lab from non-organic ingredients. Before that, holy urea could only obtained from 100% natural urine (yes,
its name is intentionally suggestive1). Since then, plenty of other natural compounds capitulated to the efforts of chemists.
But more importantly, something else came to light: Nature was holding out on us.
That is, if you look at the structures of natural compounds, it's easy to "continue the thought", and come up with other similar compounds.
Yet, Nature makes only a few of them. Curious chemists started making all those compounds that Nature never bothered to. At
that point, Organic Chemistry diverged from its roots: it boldly went when Nature has not gone before (the
science that stayed with what Nature makes, as well as chemistry happening in living organisms branched off under the name of biochemistry).
 Oh, yeah, the difference between organic and non-organic compounds turned out to be - carbon. Organic compounds tend to have a lot of it.
Roughly speaking - organic chemistry is the chemisty of carbon. Since there are 117 other currently known elements one might think that would make organic
chemistry a small niche area. But that one would be flat wrong: out of approximately 10 million currently known chemical compounds about 9 million are organic.
Oh, yeah, the difference between organic and non-organic compounds turned out to be - carbon. Organic compounds tend to have a lot of it.
Roughly speaking - organic chemistry is the chemisty of carbon. Since there are 117 other currently known elements one might think that would make organic
chemistry a small niche area. But that one would be flat wrong: out of approximately 10 million currently known chemical compounds about 9 million are organic.
So, what's so special about carbon then? Oddly, just two things:
1) It can reliably attach up to 4 other atoms
2) Any or all of these other atoms can be another carbon
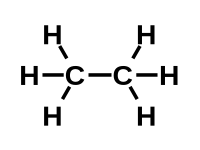 #1 is not too difficult. In fact, some elements can sometimes do a lot better. Now, #2 is a lot more rare... Somehow elements don't
seem to be too fond of their own kind. If attached to another atom of themselves, they tend to look for an opportunity to ditch it and attach to an atoms of
some other element. For instance, silicon-silicon bond is about half as strong as silicon-oxygen. So, given half a chance, silicons will split up to attach to an oxygen.
In comparison, a carbon-carbon bond is 97% as strong as carbon-oxygen (and 13% stronger than carbon-nitrogen). Still, a few elements can do #2. But none can quite do both.
#1 is not too difficult. In fact, some elements can sometimes do a lot better. Now, #2 is a lot more rare... Somehow elements don't
seem to be too fond of their own kind. If attached to another atom of themselves, they tend to look for an opportunity to ditch it and attach to an atoms of
some other element. For instance, silicon-silicon bond is about half as strong as silicon-oxygen. So, given half a chance, silicons will split up to attach to an oxygen.
In comparison, a carbon-carbon bond is 97% as strong as carbon-oxygen (and 13% stronger than carbon-nitrogen). Still, a few elements can do #2. But none can quite do both.
So, carbon is this awesome tiny lego block – you can build variety of things with it, and they will not come apart easily.
A few other elements also needed to complete that picture. Hydrogen (the most common element in the universe) serves as a plug – any bond that isn't used to attach something meaningful, can be capped off by attaching a hydrogen. Oxygen, nitrogen, sulfur and a few others drop by to add some color and variety.
Other element can be thrown in, too, but then it is usually considered to be a hybrid: a union of organic and non-organic, usually called "somethingorganic" or "organosomething", e.g. "fluoroorganic", "organometallic", etc.
And that's the story how carbon met itself. And lived happily ever after.
Note 1: That indeed used to be a popular pastime among chemists - collecting large amounts of urine and fiddling with it in the lab. We don't do that anymore. If you want to do that nowadays, you need to go to a medical lab instead.
 Organic Chemistry should be chemistry made only with natural ingredients, without those nasty chemicals, right? Well, that's not too far off. It started out as the study of stuff coming from living organisms. That stuff seemed distinctly different from the rest of chemical compounds – it was sort of softer and smoother, more "organic".
Organic Chemistry should be chemistry made only with natural ingredients, without those nasty chemicals, right? Well, that's not too far off. It started out as the study of stuff coming from living organisms. That stuff seemed distinctly different from the rest of chemical compounds – it was sort of softer and smoother, more "organic".  Oh, yeah, the difference between organic and non-organic compounds turned out to be - carbon. Organic compounds tend to have a lot of it.
Roughly speaking - organic chemistry is the chemisty of carbon. Since there are 117 other currently known elements one might think that would make organic
chemistry a small niche area. But that one would be flat wrong: out of approximately 10 million currently known chemical compounds about 9 million are organic.
Oh, yeah, the difference between organic and non-organic compounds turned out to be - carbon. Organic compounds tend to have a lot of it.
Roughly speaking - organic chemistry is the chemisty of carbon. Since there are 117 other currently known elements one might think that would make organic
chemistry a small niche area. But that one would be flat wrong: out of approximately 10 million currently known chemical compounds about 9 million are organic. #1 is not too difficult. In fact, some elements
#1 is not too difficult. In fact, some elements